6T-CEM Cells
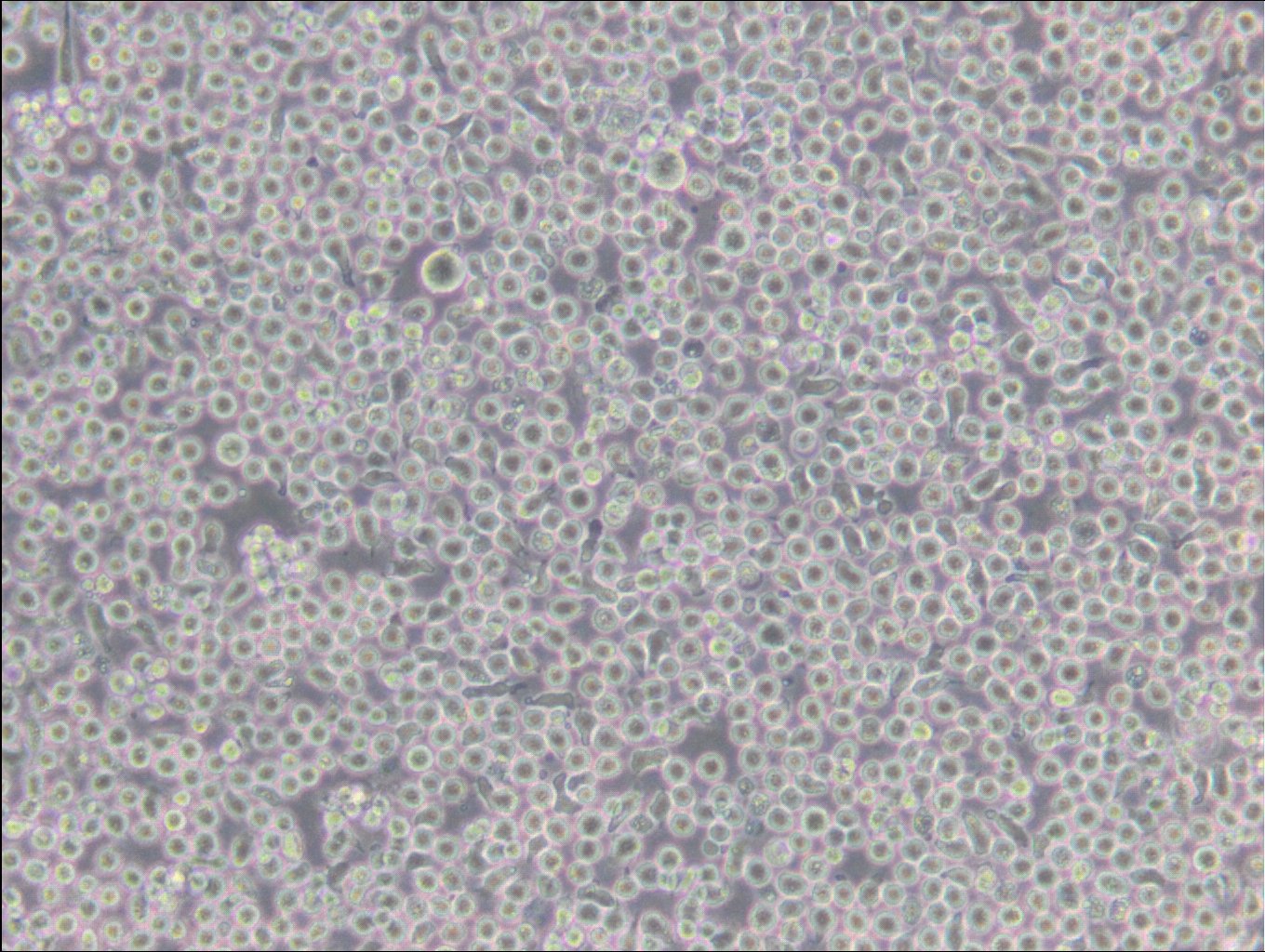
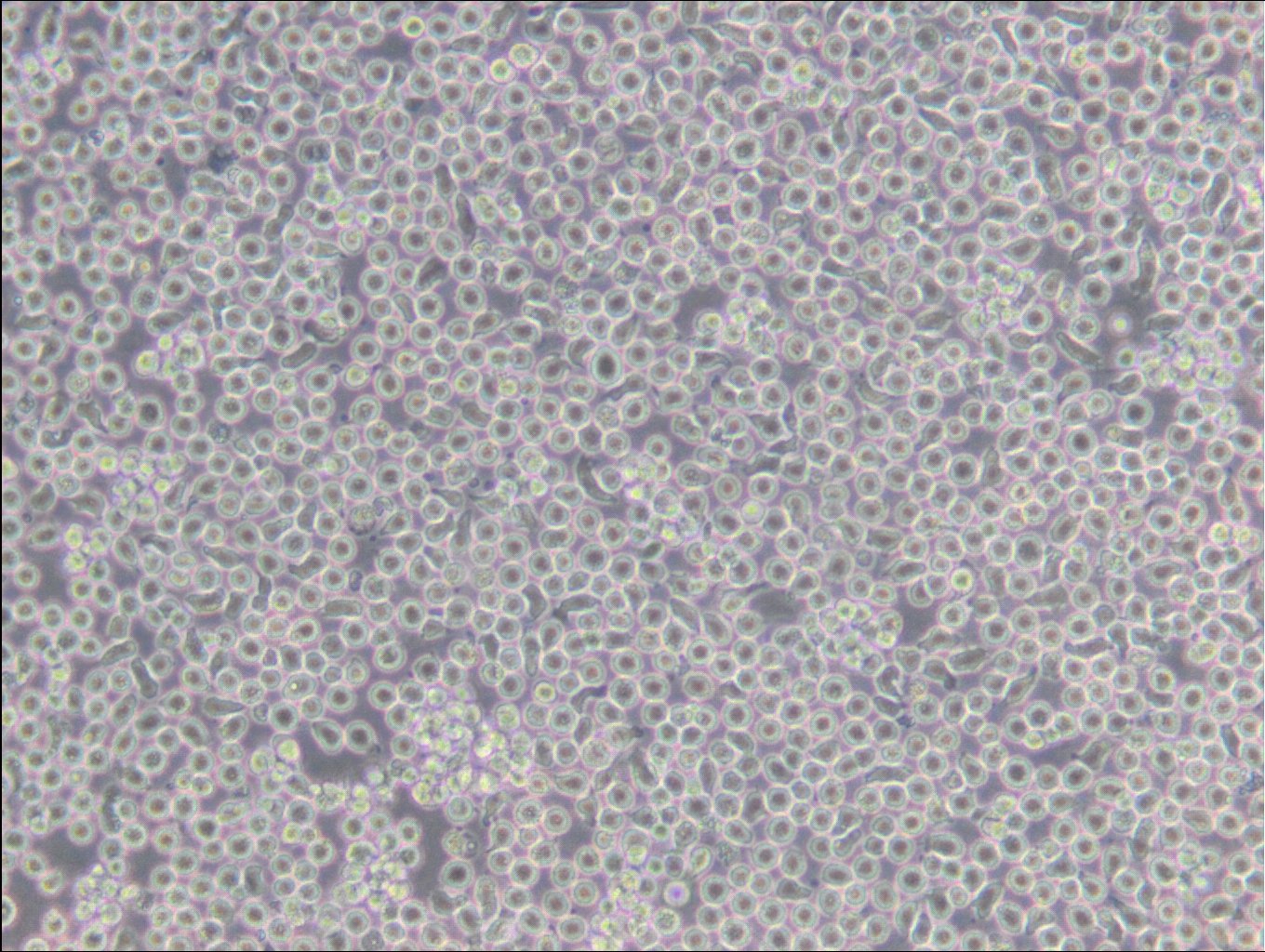









General information
| Description | The 6T-CEM cell line is a thioguanine-resistant variant of the CCRF-CEM human T-cell leukemia cell line. These cells grow in suspension and are deficient in the enzyme hypoxanthine phosphoribosyl transferase (HPRT, HGPRT) which confers resistance to thioguanine. In addition 6T-CEM are also known for secreting high levels of an immunosuppressive factor. The 6T-CEM cells have been widely used as a fusion partner for creating human T-cell hybridomas. Overall, 6T-CEM cells are a useful tool for researchers studying T-cell acute lymphoblastic leukemia, drug resistance, and the development of new treatment strategies and the molecular pathways involved in leukemogenesis, as well as for the generation of human T-cell hybridomas for immunological research. |
|---|---|
| Organism | Human |
| Tissue | Peripheral blood |
| Disease | T-cell acute lymphoblastic leukemia |
| Synonyms | 6-T CEM |
Characteristics
| Age | 4 years |
|---|---|
| Gender | Female |
| Ethnicity | Asian |
| Morphology | Lymphoblast |
| Growth properties | Suspension |
Identifiers / Biosafety / Citation
| Citation | 6T-CEM (Cytion catalog number 305132) |
|---|---|
| Biosafety level | 2 |
Expression / Mutation
Handling
| Culture Medium | Alpha MEM, w: 2.0 mM stable Glutamine, w/o: Ribonucleosides, w/o: Deoxyribonucleosides, w: 1.0 mM Sodium pyruvate, w: 2.2g/L NaHCO3 |
|---|---|
| Medium supplements | Supplement the medium with 10% FBS |
| Subculturing | Gently homogenize the cell suspension in the flask by pipetting up and down, then take a representative sample to determine the cell density per ml. Dilute the suspension to achieve a cell concentration of 1 x 10^5 cells/ml with fresh culture medium, and aliquot the adjusted suspension into new flasks for further cultivation. |
| Split ratio | 1:2 to 1:4 |
| Fluid renewal | 2 to 3 times per week |
| Freeze medium | CM-1 (Cytion catalog number 800100) or CM-ACF (Cytion catalog number 806100) |
| Handling of cryopreserved cultures |
|
Quality control / Genetic profile / HLA
| Sterility | Mycoplasma contamination is excluded using both PCR-based assays and luminescence-based mycoplasma detection methods. To ensure there is no bacterial, fungal, or yeast contamination, cell cultures are subjected to daily visual inspections. |
|---|---|
| STR profile |
Amelogenin: x,x
CSF1PO: 10,11
D13S317: 11,12
D16S539: 10,13
D5S818: 11,13
D7S820: 9,14
TH01: 6,7
TPOX: 8
vWA: 17,19
D3S1358: 15
D21S11: 31,33.2
D18S51: 13,18
Penta E: 5,14
Penta D: 11
D8S1179: 13
FGA: 23,24
D6S1043: 11,14
D2S1338: 24
D12S391: 17,18,20,21
D19S433: 14,15
|
