BV2 Cells
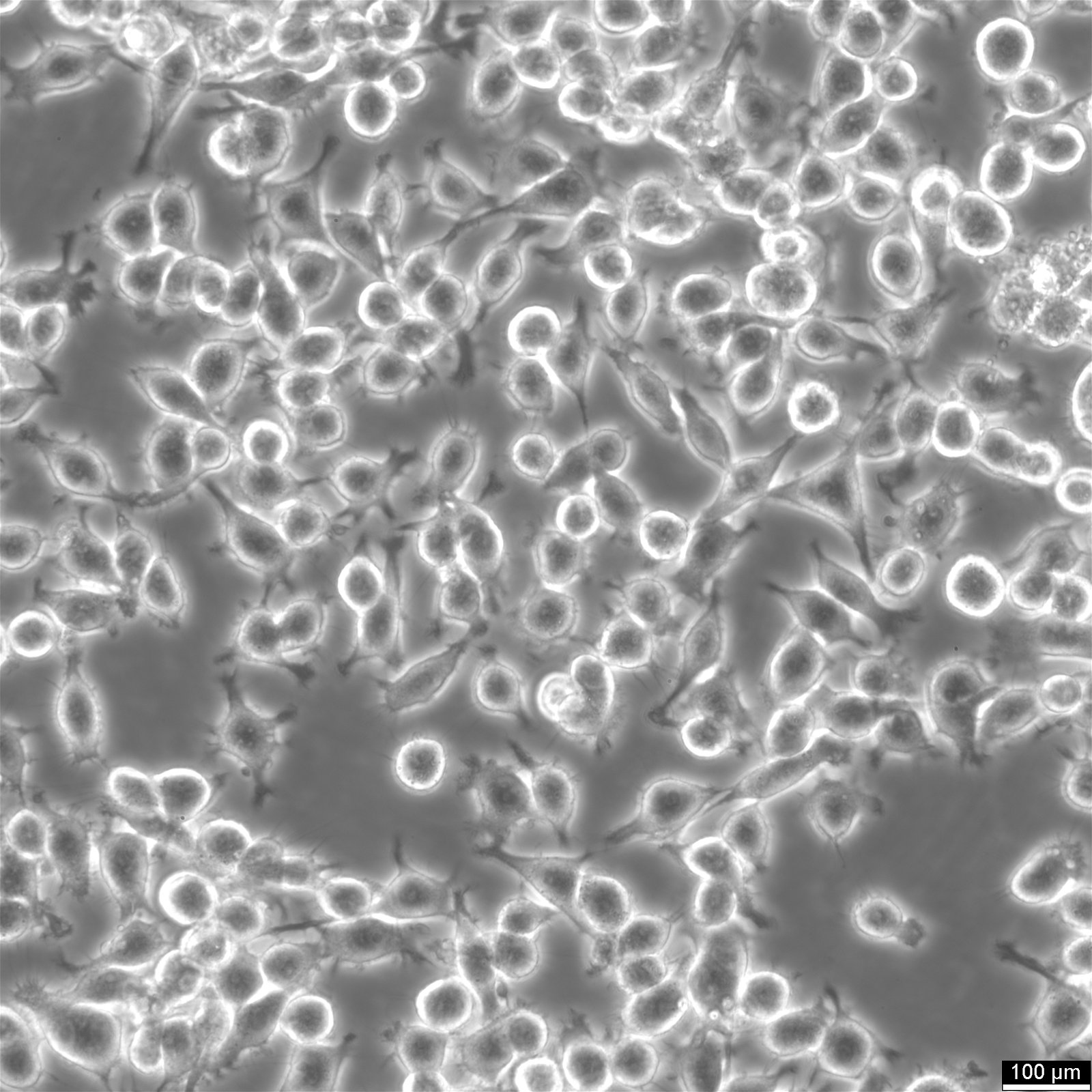
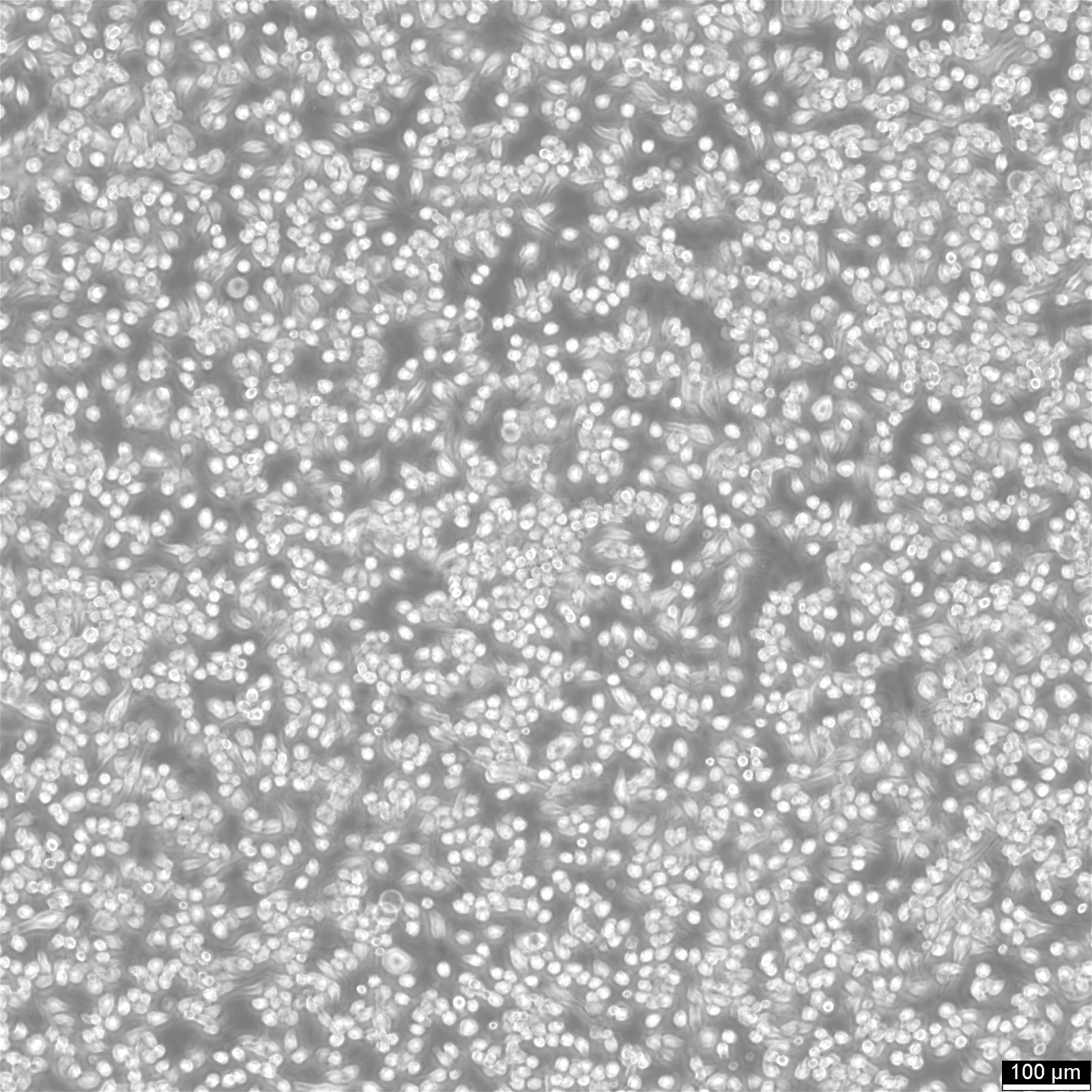
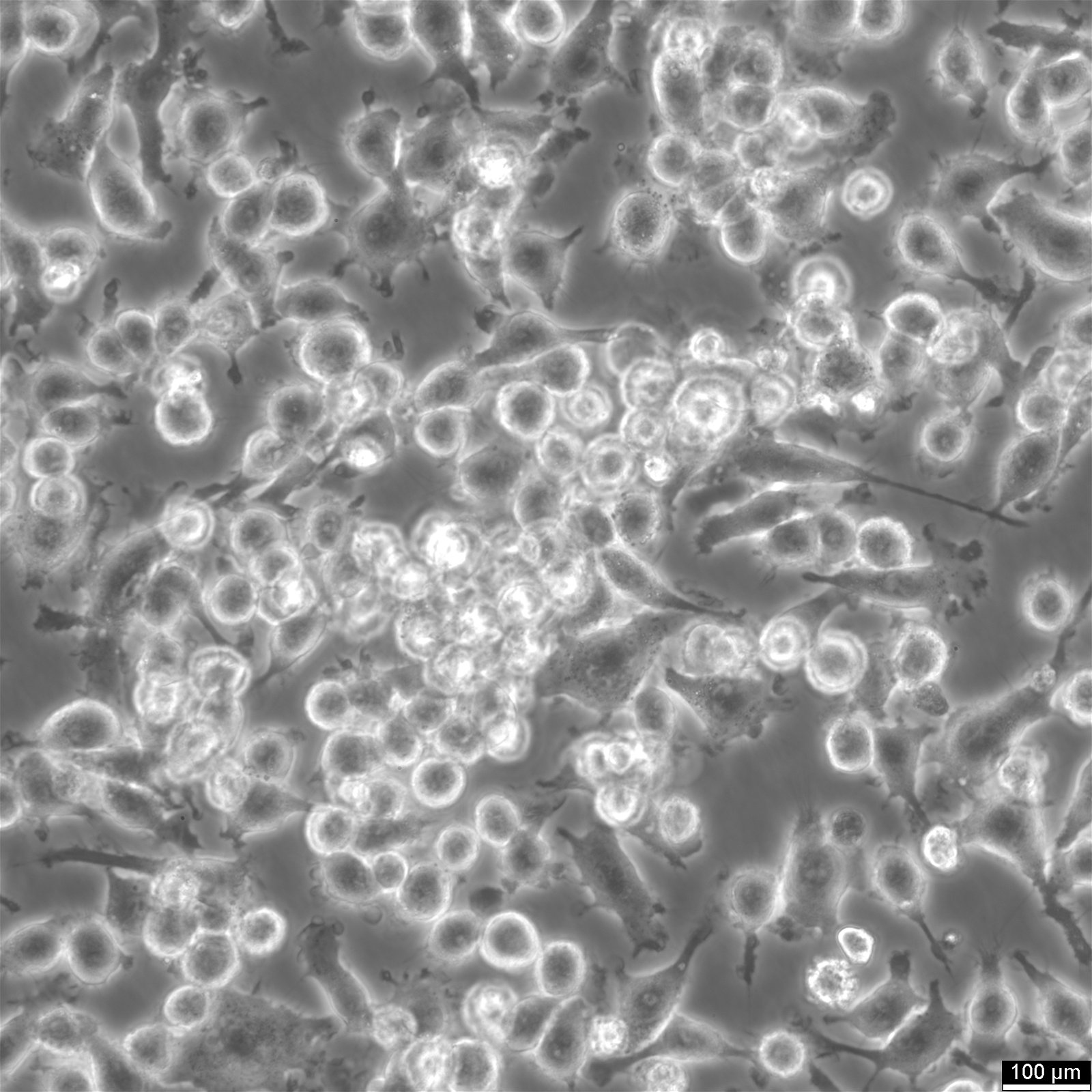









Insights on BV2 cells
| Description | BV2 cells are a type of microglial cell line derived from C57BL/6 murine, a widely used laboratory mouse strain for animal experiments. These microglial cells have been immortalized using the J2 retrovirus, which carries the v-raf and v-myc oncogenes, resulting in a stable cell line with unique characteristics. BV2 cells express nuclear v-myc and cytoplasmic v-RAF oncogenes, along with the env gp70 antigen on their surface, contributing to their role in immune responses and inflammation within the brain. One of the critical advantages of BV2 cells is their ability to retain the morphological and functional characteristics of primary microglia, the resident immune cells of the central nervous system, making them an ideal model for studying neurodegeneration and brain inflammation. The role of microglia in neurodegeneration, toxicology, and immunity, particularly in conditions such as Alzheimer's disease, is an ever-growing field in biomedical research. Traditional studies often rely on primary microglia cultures and continuous cell preparations. Using a microglia-like cell line, such as BV2 cells, offers a promising alternative by providing a continuous and reproducible source of microglia. BV2 cells, due to v-raf/v-myc expression, show enhanced metabolism and growth, ideal for research on microglial activation and inflammation. Their expression of specific oncogenes and antigens mirrors macrophages, making them valuable for studying immune responses and disease mechanisms. A recent re-evaluation of mice BV2 microglia cells examined their suitability as a substitute for primary microglia (PM). The response of BV2 cells to lipopolysaccharide was compared with that of microglia in both in vitro and in vivo settings, however, with the upregulation of genes being slightly less pronounced on average. BV2 cells displayed normal regulation of nitric oxide and functional response to IFN-gamma, critical parameters for their interaction with T cells, neurons, and other glial cells such as astrocytes. BV2 cells were also found to stimulate other glial cells effectively, leading to the production of interleukin-6 (IL-6) in astrocytes. This interaction between astrocytes and microglia is crucial for understanding the complex cell-cell interactions and the inflammatory response in the brain, especially in the context of neurodegenerative diseases like Alzheimer's, where proteins such as NAPoe31 and NAPoe41, as well as pathways like the startle response and apoptosis, play significant roles. BV2 cells offer a robust and reliable tool for researchers in microglial biology. Their expression of v-raf/v-myc oncogene products enables them to retain key characteristics of microglia and macrophages. BV2 cells have proven to be a valid substitute for primary microglia in various experimental settings, facilitating research on neurodegeneration, toxicology, immunity, and cell-cell interactions. |
|---|---|
| Organism | Mouse |
| Tissue | Brain |
| Synonyms | BV-2 |
Specifications
| Age | 1 week |
|---|---|
| Gender | Female |
| Morphology | Morphology microglial |
| Growth properties | Adherent/suspension |
Documentation
| Citation | BV2 (Cytion catalog number 305156) |
|---|---|
| Biosafety level | 2 |
Genetics of the BV2 microglia cell line
Handling
| Culture Medium | RPMI 1640, w: 2.1 mM stable Glutamine, w: 2.0 g/L NaHCO3 (Cytion article number 820700a) |
|---|---|
| Medium supplements | Supplement the medium with 10% FBS |
| Passaging solution | Accutase |
| Subculturing | Gather the suspension cells in a 15 ml tube and gently wash the adherent cells with PBS lacking calcium and magnesium (use 3-5 ml for T25 flasks and 5-10 ml for T75 flasks). Apply Accutase (1-2 ml for T25 flasks, 2.5 ml for T75 flasks) ensuring full coverage of the cell layer. Allow the cells to incubate at room temperature for 10 minutes. Following incubation, combine and centrifuge both the suspension and adherent cells. After centrifugation, carefully resuspend the cell pellet and transfer the cell suspension into new flasks containing fresh medium. |
| Split ratio | 1:2 to 1:4 |
| Fluid renewal | 2 to 3 times per week |
| Freeze medium | CM-1 (Cytion catalog number 800100) or CM-ACF (Cytion catalog number 806100) |
| Handling of cryopreserved cultures |
|
BV2 cell quality assurance
| Sterility | Mycoplasma contamination is excluded using both PCR-based assays and luminescence-based mycoplasma detection methods. To ensure there is no bacterial, fungal, or yeast contamination, cell cultures are subjected to daily visual inspections. |
|---|---|
| STR profile |
M_18-3: 16,17
M_4-2: 20.3
M_6-7: 15
M_3-2: 14
M_19-2: 13
M_7-1: 26.2
M_1-1: 16,17
M_Sex: x
M_8-1: 16
M_2-1: 16
M_15-3: 22.3,23.3,24.3
M_6-4: 18
M_11-2: 16
M_1-2: 19
M_17-2: 15
M_12-1: 17
M_5-5: 17
M_X-1: 27
M_13-1: 17
Human D4/D8: -
|
