EL4 Cells
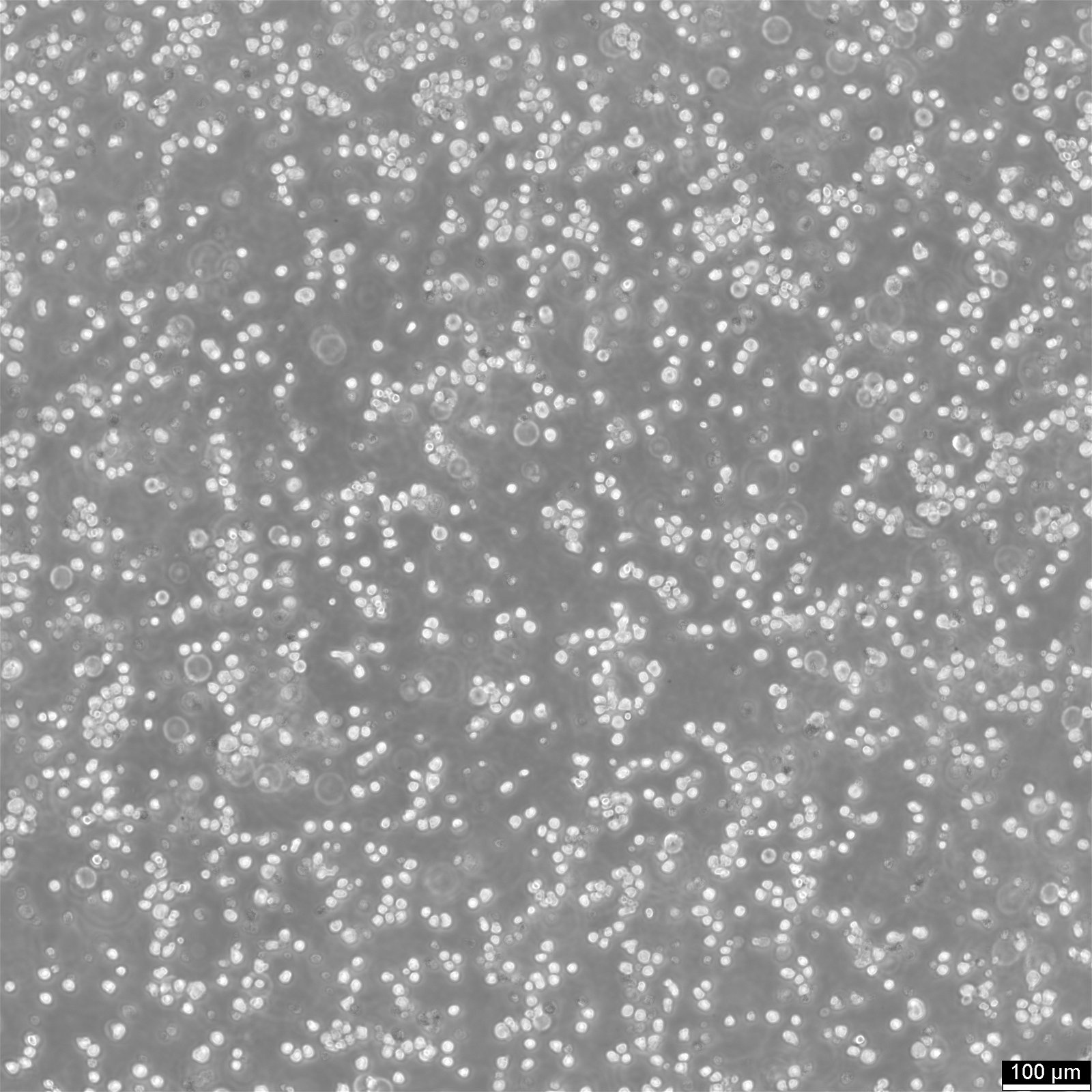

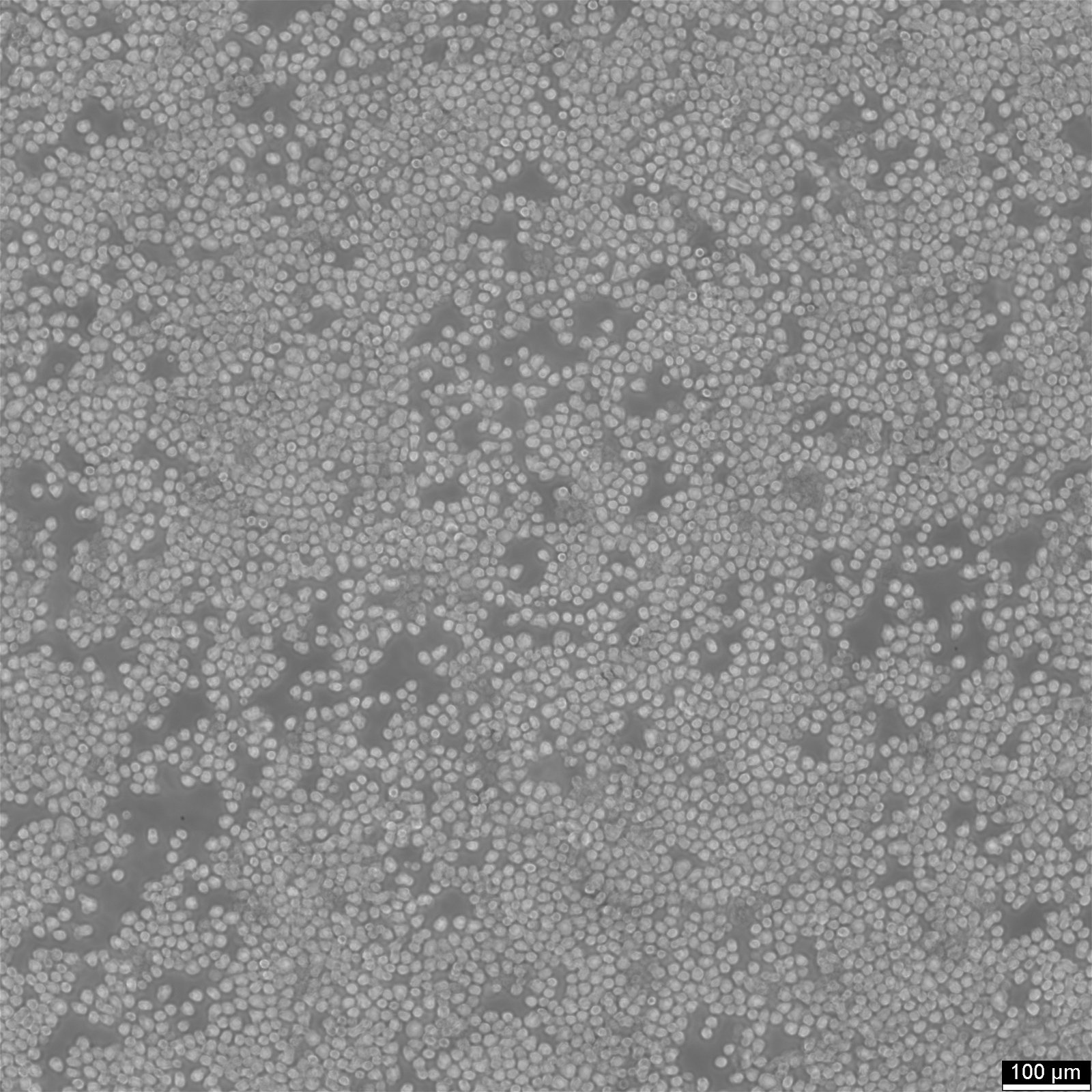
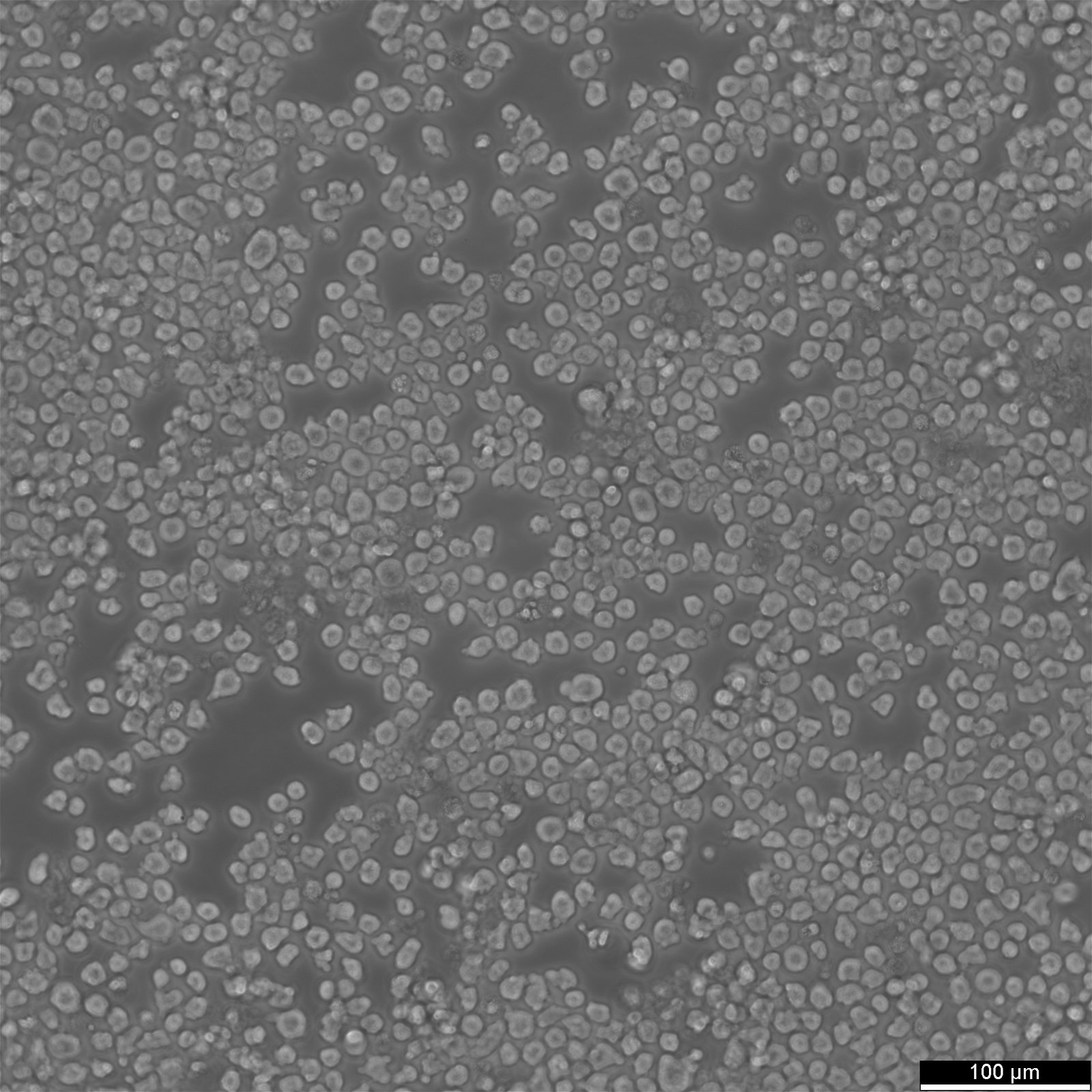












Key facts about EL4 cells
| Description | EL4 cells, derived from a lymphoma induced in a C57BL/6N mouse, are a valuable tool in immunology research and tumor immunology. The EL-4 cell line is known for its resistance to cortisol and dexamethasone and its sensitivity to phytohemagglutinin (PHA). EL4 mouse thymoma cells are used for a variety of research purposes, including studies on tumor immunology, cell signaling, apoptosis, necrosis and drug response. The origin of EL4 cells from murine thymoma makes them a relevant model for studying thymic tumors and T-cell biology, providing insights into T-cell development, thymic education, and T-cell leukemias. EL4 cells have also been used to assess the extravasation of tumor necrosis factor (TNF)-induced substances and evaluate the effectiveness of various therapeutic agents. EL4 cells have been reported to produce low levels of type G (Gross) virus antigen histone-2b (H-2b), interleukin-4 (IL-4), and Thy-1.2 antigen. EL4 cells display a complex phenotype with mosaic expression of several surface molecules, including CD2, CD3, CD4, CD32, and CD44. Particularly interesting is the linkage between CD3 and CD2 expression, suggesting a coordinated regulation of these molecules. The use of EL4 cells in xenograft models involves transplanting these cells into immunodeficient mice to study tumor growth and the in vivo efficacy of anti-cancer therapies. In summary, EL4 cells are a versatile mouse lymphoma cell line used extensively in immunological and cancer research, known for their complex and variable expression of surface molecules. |
|---|---|
| Organism | Mouse |
| Disease | Mouse precursor T cell lymphoblastic lymphoma/leukemia |
| Applications | Cancer research, 3D cell culture, Immunology |
| Synonyms | EL-4, EL 4, E.L.4 |
Details of the murine cell line EL4
| Age | Unspecified |
|---|---|
| Gender | Unspecified |
| Morphology | Lymphoblast |
| Cell type | T lymphoblast |
| Growth properties | Suspension |
Identifiers / Biosafety / Citation
| Citation | EL4 (Cytion catalog number 300653) |
|---|---|
| Biosafety level | 1 |
Genotype of EL-4 cells
| Surface antigens | H-2b, Thy-1.2 |
|---|---|
| Viruses | MLV +, Negative for ectromelia virus (mousepox) |
| Karyotype | modal number = 39 |
Handling
| Culture Medium | RPMI 1640, w: 2.1 mM stable Glutamine, w: 2.0 g/L NaHCO3 (Cytion article number 820700a) |
|---|---|
| Medium supplements | Supplement the medium with 10% FBS |
| Subculturing | Gently homogenize the cell suspension in the flask by pipetting up and down, then take a representative sample to determine the cell density per ml. Dilute the suspension to achieve a cell concentration of 1 x 10^5 cells/ml with fresh culture medium, and aliquot the adjusted suspension into new flasks for further cultivation. |
| Freeze medium | CM-1 (Cytion catalog number 800100) or CM-ACF (Cytion catalog number 806100) |
| Handling of cryopreserved cultures |
|
Quality control
| Sterility | Mycoplasma contamination is excluded using both PCR-based assays and luminescence-based mycoplasma detection methods. To ensure there is no bacterial, fungal, or yeast contamination, cell cultures are subjected to daily visual inspections. |
|---|
